


1. All molecules are particular combinations of atoms.
The atomic parts of molecules are shown in a chemical formula.
There are patterns to the properties of atoms, and they influence, but are not the same as, molecular properties. If you want to review the chemical formulas and atomic properties, you can explore periodic tables at the folowing web sites:
http://www.whfreeman.com/chemistry3e/CHAP.HTML and http://www.ch.ic.ac.uk/motm/
http://www.shef.ac.uk/chemistry/chemdex/chem-periodic-tables.html ( periodic tables in many languages)
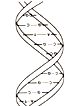
2. There are millions of different molecules."It is thought some twelve million molecules have been characterised and reported by scientists. Some are found only in nature; others have only existed in scientific laboratories, perhaps only for fractions of a second. Some molecules consist of only a few atoms of one or more of the chemical elements, others have a few thousand atoms, whilst "polymers" may contain millions.
The vast majority of molecules contain carbon atoms, and are known as "organic", those associated with living organisms sometimes have the appelation "bio-organic", whilst those without at least one carbon and one hydrogen atoms are known as "inorganic" species. "-- Dr. Henry Rzepa in the Department of Chemistry at Imperial
College.
3. Molecules vary in weight and size. Organic molecules, for the most part,can be rather large. Vitamin B2, for example, is one of the smallest, and hemoglobin one of the largest organic molecule.
4. Molecules can be represented in different ways.
A "ball and stick" model represents the size of a molecule with real relative distances among atoms.
But what about the balls? Are they the right relative size? Not really.
A "space-filling" model represents the approximate space that the atoms take up.